Study design
ALPINE was a phase 3, randomised study of BRUKINSA®▼ compared with ibrutinib in patients with relapsed/refractory (R/R) chronic lymphocytic leukaemia (CLL) (Figure 1).1,2
Figure 1. ALPINE: Study design.1,2
Patients
The ALPINE study enrolled patients with R/R CLL after at least one prior systemic therapy.1–3
Stratification
Randomisation was stratified by age (<65 years versus ≥65 years), geographic region (China versus non-China), refractory status (yes or no), and del(17p)/TP53 mutation status (present or absent).1–3
Key baseline characteristics
The key baseline characteristics are summarised below in Table 1.
Table 1. ALPINE: key baseline characteristics (ITT population).1*
| Characteristic | BRUKINSA (n=327) | Ibrutinib (n=325) |
|---|---|---|
| Median age, years (range) ≥75 yr | 67 (35–90) 74 (22.6) | 68 (35–89) 69 (21.2) |
| Male, n (%) | 213 (65.1) | 232 (71.4) |
| Disease stage, n (%) Binet stage A/B or Ann Arbor stage I/II Binet stage C or Ann Arbor stage III/IV | 182 (55.7) 145 (44.3) | 189 (58.2) 135 (41.5) |
| ECOG performance status, n (%) ≥1† | 198 (60.6) | 203 (62.5) |
| Median prior lines of therapy (range) ≥3 prior lines, n (%) | 1 (1–6) 24 (7.3) | 1 (1–12) 30 (9.2) |
| Prior chemoimmunotherapy, n (%) | 260 (79.5) | 247 (76.0) |
| Bulky disease (≥5 cm), n (%)‡ | 145 (44.3) | 149 (45.8) |
| del(17p) and/or mutated TP53, n (%) TP53 mutated, n (%) | 45 (13.8) 30 (9.2) | 50 (15.4) 25 (7.7) |
| del(11q), n (%) | 91 (27.8) | 88 (27.1) |
| IGHV mutation Mutated Unmutated | 79 (24.2) 239 (73.1) | 70 (21.5) 239 (73.5) |
| Complex karyotype§ | 56 (17.1) | 70 (21.5) |
Primary endpoint
The primary endpoint in the ALPINE study was the investigator-assessed (IA) ORR, defined as complete response (CR) or partial response (PR), which was met at a pre-planned interim analysis.1,4
A pre-planned interim analysis of the first 415 enrolled patients was conducted after a median follow up of 15.3 months.3,4 IA-ORR (partial or complete response) was significantly higher with BRUKINSA (78.3%; 95% confidence interval [CI]: 72.0, 83.7) versus ibrutinib (62.5%; 95% CI: 55.5, 69.1), demonstrating non-inferiority (one-sided p<0.0001) and superiority (two-sided p=0.0006) of BRUKINSA to ibrutinib.3,4 A consistent benefit in favour of BRUKINSA was observed across all pre-specified patient subgroups.4
IA-ORR continued to be higher in the BRUKINSA group compared with the ibrutinib group in subsequent analyses; 79.5% (95% CI: 74.7, 83.8) versus 71.1% (95% CI: 65.8, 75.9) at 24 months (descriptive p=0.0133) and 83.5% (95% CI: 79.0, 87.3) and 74.2% (95% CI: 69.0, 78.8) in the final analysis, respectively.1,3,5 Similar results were observed when ORR was assessed by an independent review committee.1,3,4
At a median follow-up of 39.0 months, ORR remained higher in the BRUKINSA group compared with the ibrutinib group (90% versus 83%, respectively) at 48 months. While responses deepened over time in both treatment groups, a higher proportion of patients in the BRUKINSA cohort achieved CR/CR with incomplete recovery compared with the ibrutinib group.2
Secondary endpoint
IA-PFS was a key secondary endpoint in the ALPINE study.1,2 At a median follow-up of 39.0 months, BRUKINSA was superior to ibrutinib for PFS (hazard ratio 0.68 (95% CI: 0.53, 0.86); p=0.0011 (two-sided descriptive).1,2
Figure 2. Extended follow-up: Superior PFS with BRUKINSA versus ibrutinib2
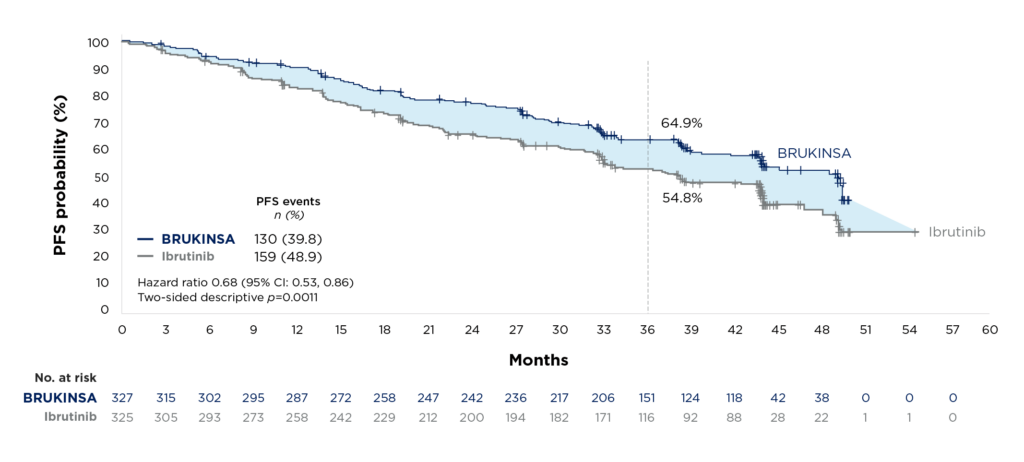
The PFS benefit in favour of BRUKINSA was observed across major patient subgroups, including patients with high-risk cytogenetics (del(17p)/TP53 mutation).2
Safety
The overall median treatment duration was 38.3 months in the BRUKINSA group and 35.0 months in the ibrutinib group.2
With a median follow-up of 39.0 months, a total of 98.8% (320/324) of patients in the BRUKINSA group and 99.7% (323/324) of patients in the ibrutinib group experienced an adverse event.2 Serious adverse events occurred in 50.9% (165/324) of patients in the BRUKINSA group and 59.0% (191/324) of patients in the ibrutinib group.2
The most common adverse events of any grade that occurred in at least 15% of patients in either group included anaemia,* arthralgia, diarrhoea, upper respiratory tract infection, hypertension,* neutropenia,* and COVID-19 related* (includes preferred terms of COVID-19, COVID-19 pneumonia, and suspected COVID-19).2
*Pooled MedDRA preferred terms.
Atrial fibrillation and flutter (any grade; a key secondary endpoint) occurred in 6.8% (22/324) of patients in the BRUKINSA group and 16.4% (53/324) of patients in the ibrutinib group.1,2
The overall safety/tolerability summary, adverse events of special interest, and the cardiac safety profile of BRUKINSA are summarised in Tables 2, 3, and 4, respectively.
Table 2. ALPINE: Overall safety/tolerability summary at a median follow-up of 39 months2
| Event, n (%) | BRUKINSA (n=324) | Ibrutinib (n=324) |
|---|---|---|
| Median treatment duration, months | 38.3 (0.4, 54.9) | 35.0 (0.1, 58.4) |
| Any grade adverse event | 320 (98.8) | 323 (99.7) |
| Grade 3 to 5 adverse events | 235 (72.5) | 251 (77.5) |
| Grade 5 adverse events | 41 (12.7) | 40 (12.3) |
| Serious adverse event | 165 (50.9) | 191 (59.0) |
| Adverse events leading to | ||
| Dose reduction | 47 (14.5) | 59 (18.2) |
| Dose interruption | 196 (60.5) | 201 (62.0) |
| Treatment discontinuation | 64 (19.8) | 85 (26.2) |
| Hospitalisation | 150 (46.3) | 180 (55.6) |
Table 3. ALPINE: Adverse events of special interest at a median follow-up of 39 months2
| Adverse events of special interest,* n (%) | BRUKINSA (n=324) | Ibrutinib (n=324) | ||
|---|---|---|---|---|
| Any grade | Grade ≥3 | Any grade | Grade ≥3 | |
| Infection | 264 (81.5) | 115 (35.5) | 260 (80.2) | 111 (34.3) |
| Opportunistic Infections | 8 (2.5) | 6 (1.9) | 13 (4.0) | 5 (1.5) |
| COVID-19 Related† | 145 (44.8) | 56 (17.3) | 105 (32.4) | 38 (11.7) |
| Bleeding | 142 (43.8) | 12 (3.7) | 144 (44.4) | 13 (4.0) |
| Major Haemorrhage | 13 (4.0) | 12 (3.7) | 16 (4.9) | 13 (4.0) |
| Hypertension | 86 (26.5) | 53 (16.4) | 80 (24.7) | 47 (14.5) |
| Atrial fibrillation/flutter | 22 (6.8) | 10 (3.1) | 53 (16.4) | 16 (4.9) |
| Anaemia | 53 (16.4) | 7 (2.2) | 59 (18.2) | 11 (3.4) |
| Neutropenia | 100 (30.9) | 72 (22.2) | 94 (29.0) | 72 (22.2) |
| Thrombocytopenia | 43 (13.3) | 12 (3.7) | 53 (16.4) | 19 (5.9) |
| Second primary malignancies | 46 (14.2) | 26 (8.0) | 52 (16.0) | 19 (5.9) |
*Pooled MedDRA preferred terms; †Includes preferred terms of COVID-19, COVID-19 pneumonia, and suspected COVID-19. Adapted from Brown JR, et al. ASH. 2023.2
Table 4. ALPINE: Cardiac safety profile at a median follow-up of 39 months2
| Event, n (%) | BRUKINSA (n=324) | Ibrutinib (n=324) |
|---|---|---|
| Cardiac adverse events | 80 (24.7) | 112 (34.6) |
| Serious cardiac adverse events | 11 (3.4) | 31 (9.6) |
| Cardiac adverse events leading to treatment discontinuation | 3 (0.9) | 15 (4.6) |
| Fatal cardiac events | 0 | 6 (1.9)* |
Patient-reported outcomes (PROs)
Health-related Quality of life (HRQoL) outcomes were assessed in the ALPINE study using the patient-reported outcome measures EORTC QLQ-C30 and EQ-5D-5L.6,7
Treatment with BRUKINSA resulted in clinically meaningful improvements in HRQoL, including EORTC QLQ-C30 GHS and functioning scales and symptom scales.7 These improvements were maintained over time.7 No significant differences were observed between the two treatment arms, however, it should be noted that HRQoL was generally good at baseline.7
