1st November 2022 3:00PM GMT - Virtual
The changing landscape of Waldenström’s Macroglobulinemia (WM)
The purpose of this meeting was to discuss the use of BRUKINSA®▼ (zanubrutinib), a next generation BTK inhibitor, in clinical practice and start the conversation about its place in the patient’s treatment pathway and in hospital protocols. The aim was to facilitate the exchange of best practice and expert opinion in order to benefit patients and provide value to healthcare professionals.
AGENDA
Recordings
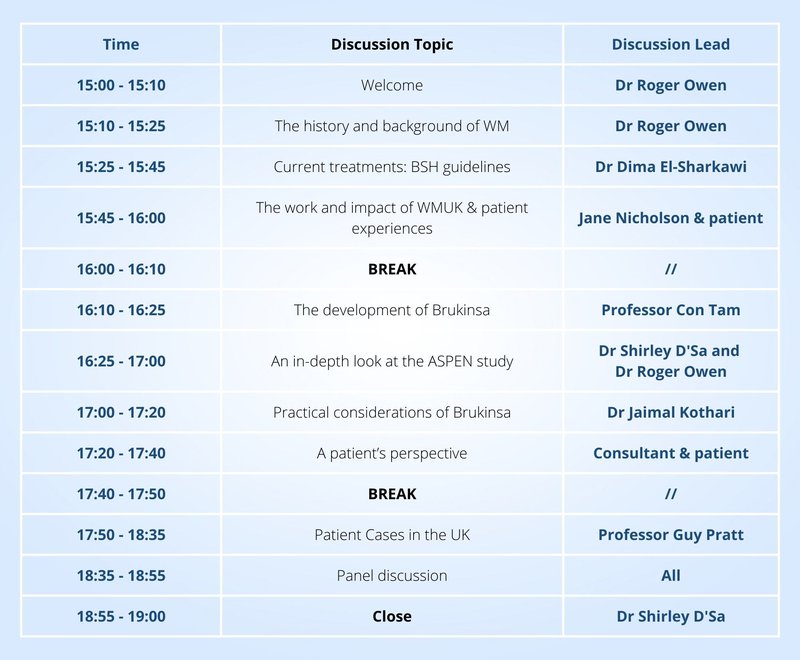
Diagnosis, Clinical Features and Indications for WM Therapy
An In-Depth Look at the ASPEN Study – Dr Roger Owen and Dr Shirley D’Sa
Practical Considerations of BRUKINSA – Dr Jaimal Kothari
Current Treatments: BSH Guidelines – Dr Dima El-Sharkawi
Speakers

Dr Shirley D’Sa
UCLH Centre for Waldenström’s Macroglobulinaemia (WM)
Shirley D’Sa, MD, FRCP, FRCPath, is a Consultant Haematologist and Clinical Lead for the UCLH Centre for Waldenström’s macroglobulinemia (WM) and Related Conditions, and Honorary Associate Professor at the UCL Cancer Institute. She is also haematological lead in the Joint Neurohaematology Service at the National Hospital for Neurology and Neurosurgery, Queen Square, London with Prof Michael Lunn a specialist in peripheral nerve diseases.

Dr Dima El-Sharkawi
The Royal Marsden NHS Foundation Trust
Dr Dima El-Sharkawi is a haematology consultant at the Royal Marsden Hospital. Her specialist interest is lymphoma, CLL and the mature lymphoid leukaemias. She is clinical lead for the SIHMDS at the Royal Marsden Hospital. She is the lead for the clinical advisory group for WMUK and is an author on the recently published guidelines for the diagnosis and management of Waldenstrom Macroglobulinaemia.

Dr Jaimal Kothari
Oxford University Hospitals
Jaimal Kothari is a consultant haematologist based at Oxford University Hospitals and an Honorary Senior Lecturer in Haematology at The University of Oxford. He completed his haematology training at University College London Hospital, and has particular interests in Waldenstroms Macroglobulinaemia and multiple myeloma. He is Chief Investigator for the UK wide WM-Pembro clinical trial, for patients with relapsed WM and a senior author on the recently published BCSH guidelines for WM.

Jane Nicholson
WMUK (Waldenström’s Macroglobulinaemia UK)
WMUK CEO

Dr Roger Owen
Leeds Teaching Hospitals NHS Trust
Consultant Haematologist at St. James University Hospital Institute of Oncology in Leeds, UK. He has a special interest in indolent lymphoma including Waldenstroms macroglobulinaemia and marginal zone lymphoma.

Professor Guy Pratt
University Hospitals Birmingham NHS Foundation Trust
Guy Pratt is a Consultant Haematologist in Birmingham with a strong clinical and research interest in Multiple Myeloma, Waldenstroms Macroglobulinaemia and related plasma cell disorders. He has an honorary title of Professor of Haematology from the University of Birmingham. He was clinical lead for the 2016 NICE myeloma guidelines and has had a number of positions nationally including vice-chair of BSH guidelines, ex-chair of the BSH science and publication committee.

Professor Constantine (Con) S. Tam
Monash University
Professor Con Tam is Head of the Lymphoma Service, Alfred Hospital and Professor of Haematology, Monash University. After his training in Haematology and Haematopathology, Con completed a Leukemia Fellowship at MD Anderson Cancer Centre in USA. Con is passionate about developing new therapies for the treatment of leukemias and lymphomas. He has published 240 peer-reviewed papers and his work has been cited >17,000 times.
