Guidelines
After a confirmed diagnosis of Waldenström’s macroglobulinemia (WM), an assessment of burden of disease and screening for complications is recommended to inform if there is an indication for therapy (Figure 1).1
Figure 1. BSH treatment algorithm.1
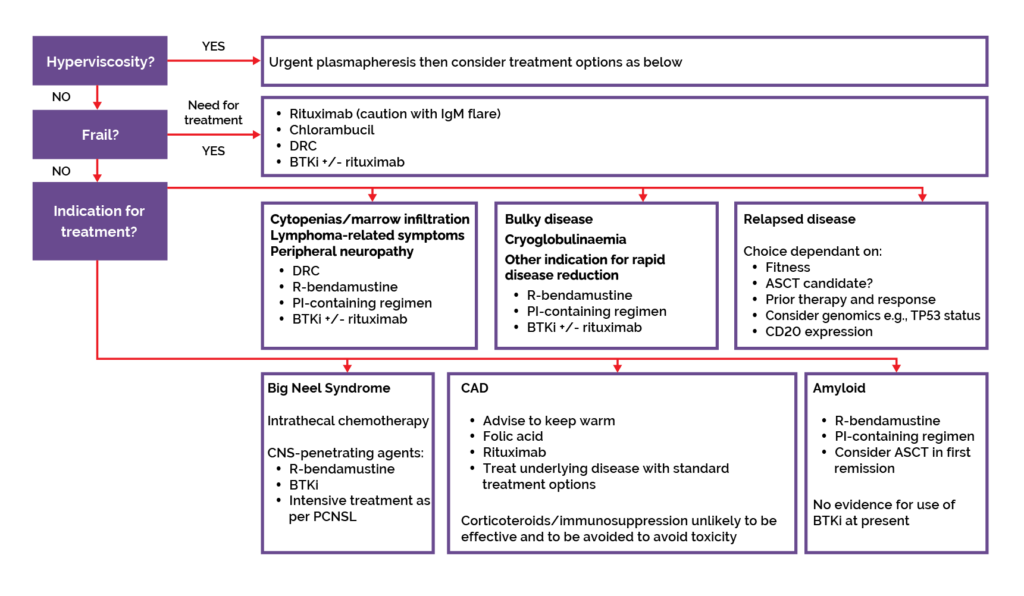
Treatment options for frontline therapy*:1
- BTK inhibitor +/- rituximab
- Dexamethasone, rituximab, cyclophosphamide (DRC)
- Proteasome inhibitor-containing regimen
- Rituximab-bendamustine (BR)
Treatment options for relapsed/refractory therapy:1
- As above – choice of therapy is dependent on previous therapies, time from prior therapy and patient factors
According to NICE guidance, BRUKINSA®▼ is recommended as a treatment option for WM in adults in the UK who have had at least one treatment, and only if bendamustine plus rituximab is also suitable.2
According to SMC guidance, BRUKINSA is accepted for use within NHS Scotland in line with its indication (for adult patients with WM who have received at least one prior therapy, or in first line treatment for patients unsuitable for chemotherapy).3
The Health Service Executive (HSE) in Ireland has also approved reimbursement for BRUKINSA for the treatment of adult patients with WM who have received at least one prior therapy, or as first-line treatment for patients unsuitable for chemo-immunotherapy.4
*The BSH guidelines are based on published evidence rather than what is funded in the UK.1 Listed in alphabetical order rather than order of preference.1 Choice of therapy will be partly based on indication for therapy and speed with which disease burden or IgM paraprotein needs to be reduced.1
Abbreviations: BSH, British Society for Haematology; BTK, Bruton’s tyrosine kinase inhibitor; IgM, immunoglobulin M; NICE, National Institute of Health and Care Excellence; SMC, Scottish Medicines Consortium.
